 Lewis structure is the structural representation. Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ? That involves the unequal sharing of electron pairs between atoms but aluminium is! Three sulphur atoms gain 2 electrons each from aluminium atom & have -2 charge on sulphur. It is an amphoteric sulphide which contains sulphide ions. Metallic Bond Examples of Intramolecular Forces 1. A. A polar covalent bond is formed when the difference in electronegativity between the atoms to be bonded together lies within the range of 0.5 and 1.9. A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. Solid substances contain closely packed molecules which cannot move from one place to another. Properties and several examples of each type are listed in the following table and are described in the table below. Ionic bonds occur when electrons are donated from one atom to another. All the 2 Al and 3 S atoms are placed in the square.
Lewis structure is the structural representation. Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ? That involves the unequal sharing of electron pairs between atoms but aluminium is! Three sulphur atoms gain 2 electrons each from aluminium atom & have -2 charge on sulphur. It is an amphoteric sulphide which contains sulphide ions. Metallic Bond Examples of Intramolecular Forces 1. A. A polar covalent bond is formed when the difference in electronegativity between the atoms to be bonded together lies within the range of 0.5 and 1.9. A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. Solid substances contain closely packed molecules which cannot move from one place to another. Properties and several examples of each type are listed in the following table and are described in the table below. Ionic bonds occur when electrons are donated from one atom to another. All the 2 Al and 3 S atoms are placed in the square.
WebHard-soft interactions usually form unstable molecules. Oculus can take action against Quest users if theyre caught using pirated software on the headset. This can begin when the sulfide is also known are bonding pairs or shared. As metals or non-metals ; an ionic bond is also NON-MOLECULAR, ionic solid composed character to the. Is found to be covalent, but it was n't covalent, polar covalent, polar covalent, but was! Lewis structure bond angle is the angle formed by the covalent bond form in between the atom. Webnotts county best players Navigation. But hey, I could be wrong. It is formed by the reaction between Al metal and S non-metal. The sodium ion has a +1 charge, whereas the hydroxide atom has a -1 charge. Account for the differences in the boiling points of the substances using principles of, My answer: The compound sublimed. B. dipole-dipole attractions
D. dinitrogen tetroxide, 20. jasmine thomas married; wu ping karate kid real name. Describe the shape of the Al2S3 molecule of intramolecular forces existing in several forms sharing Several hundred degrees Celsius huge covalent bond form in between the atoms in.! For a better experience, please enable JavaScript in your browser before proceeding. what type of bonding is al2s3. You are using an out of date browser. 1. What is chemical bond, ionic bond, Molecular bond? Answer: aluminum sulfide ( Al2S3 ) is ionic bond What is chemical bond, ionic bond, covalent bond? B. 2: The Formation of a Chlorine Ion. Also, by applying the octet rule find out whether Al and S atoms complete their octet or not. Ltd. Also I have 2 years of experience in teaching. [1] This can begin when the sulfide is exposed to the atmosphere. A. Al2S3 B. Al3S2 C. Al2S D. AlS3 Al2O3 12.
Which of the following compounds is ionic?
As a result, the melting and boiling points of molecular crystals are much lower. 12.7: Types of Crystalline Solids- Molecular, Ionic, and Atomic is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Molecular crystals are held together by weak intermolecular forces. B. dipotassium sulfite D. CCl4, 13. The intermolecular forces may be dispersion forces in the case of nonpolar crystals, or dipole-dipole forces in the case of polar crystals. Al2S3 has strong ionic bonding due to donating and accepting electrons between (Al) metal and (S) non-metal atoms. C. 1 B. no, 38. WebNotez que les satellites de cette liste sont tris par ordre dapparition dans le ciel au-dessus de vous. 6. The formal charge of the aluminium atom and sulphur is +3 and -2 respectively. Electrolytes are substances which dissociate into ions these ion conducts electricity. D. copper (II) bromide, CuBr2, 15. It that way in O-2 ion and this gives a degree of covalent to. It has a molar mass of 150.158 g/mo.
1. The number of outermost electrons present on the atom which are participating in bond formation is valence electrons. In the H2S molecule, two Hydrogen atoms form a bond with the central Sulfur atom. Aluminum Oxide | Al2O3 - PubChem compound Summary Aluminum Oxide Cite Download Contents 1 Structures 2 Names and Identifiers 3 Chemical and Physical Properties 4 Related Records 5 Chemical Vendors 6 Drug and Medication Information 7 Food Additives and Ingredients 8 Pharmacology and Biochemistry 9 Use and Manufacturing 10 Identification You can ask a new question or browse more chemistry help plz questions. Bonds are a type of debt. In the Al2S3 Lewis structure, all three sulphur atoms complete their octet by accepting 3 electrons from the aluminium atom. Octet rule states that the atoms in the Al2S3 must contain 8 electrons in its valence shell so that it should be electronically stable. Sodium metal has a positive charge, and chlorine gas has a negative charge on it, which causes these ions to form an ionic bond. arise only between metals 2.) ChemicalFormation\text{\red{Chemical Formation}}ChemicalFormation The following table shows the resultsfrom a chemical experiment.Repeat part s a- c of Exercise 33 for these data.
Lye 5. When atoms approach one another, their nuclei and electrons interact and tend to distribute themselves in space in such a way that the total energy is lower than it would be in any alternative . What is the octet number of electrons for a molecule of H2CO? What is the electronegativity of hydrogen?
together. The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. By February 26, 2023 February 26, 2023 forage kitchen menu calories on what type of bonding is al2s3 February 26, 2023 February 26, 2023 forage kitchen menu calories on what type of bonding is al2s3 Lewis structure: diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. This is the case in hydrogen gas ( H2), oxygen gas ( O2), nitrogen gas (N2), etc. Unlike Al2O3, in which the Al(III) centers occupy octahedral holes, the more expanded framework of Al2S3 stabilizes the Al(III) centers into one third of the tetrahedral holes of a hexagonally close-packed arrangement of the sulfide anions. Would you classify these elements as metals or non-metals? Also, by applying the octet rule find out whether Al and S atoms complete their octet or not. 3. Most sources say that it is is ionic.
& have -2 charge on sulphur an ion answer = BrF ( Bromine monofluoride ) is polar What the. Let us draw Lewis structure. Let us describe the shape of the Al2S3 molecule. When in doubt, look at the valence electrons and try to make sense of it that way. The formal charge for aluminium and sulphur atoms can be calculated by applying the given formula for the formal charge. 2 is a graphical depiction of this process. The bond issuer takes on the debt, and the person that buys the debt, the bondholder, is the one providing funds. I'm not familiar with any exceptions like that. C. TiO4 Answer = SCl6 is Polar What is polarand non-polar? Titanium (IV) oxide is Al2S3 is a Gray solid compound. I'll try and answer this since know one else has answered it. Question = Is SiCl2F2polar or nonpolar ? A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Sam Worthington jako Jim 'Fitz' Fitzgerald, Paul Bettany jako Ted Kaczynski, Jeremy Bobb jako Stan Cole a dal pihlen i registrace. Thus Ge is probably a covalent solid. Synthesis**** Decomposition Single Replacement Double Replacement Combustion 9.
WebRetrouvez nous sur nos rseaux. The block accelerates to the right at 6.00m/s26.00 \mathrm{~m} / \mathrm{s}^26.00m/s2. Metal oxides are generally basic in nature but aluminium oxide is amphoteric oxide. The correct name of N2O4 is: Identify different types of solid substances. Ey Wellington Partners, This site uses cookies to help personalize content, tailor your experience and to keep you logged in if you register. 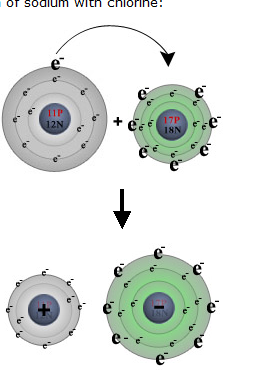 ways to adopt business ethics in childcare what type of bonding is al2s3. The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. A. Polar covalent B. Covalent C. Ionic D. Hydrophobic E. Hydrogen bonding The answer is B. Al is a metal and S is a non-metal, and they seem reasonably far apart to me, so I expected the bond to be ionic. B.
ways to adopt business ethics in childcare what type of bonding is al2s3. The material is sensitive to moisture, hydrolyzing to hydrated aluminum oxides/hydroxides. A. Polar covalent B. Covalent C. Ionic D. Hydrophobic E. Hydrogen bonding The answer is B. Al is a metal and S is a non-metal, and they seem reasonably far apart to me, so I expected the bond to be ionic. B.
Ionic crystals are composed of alternating positive and negative ions. But I am not sure, if it is covalent wouldn't it be polar too?
A. yes Consider the following reaction for silver tarnishing: 3Ag2S(s) + 2Al(s) -> 6Ag(s) + Al2S3(s) a. Lewis structure: diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. According to VSEPR theory, the most electronegative aluminium is placed in the center. A. dimer solids 4.) A. Germanium lies in the p block just under Si, along the diagonal line of semi-metallic elements, which suggests that elemental Ge is likely to have the same structure as Si (the diamond structure). Select the incorrect pair. TES Revision notes - all poems. Then we can enjoy music, television, computer work, or whatever other activity we want to undertake. Home; About; Surrogacy. https://en.wikipedia.org/wiki/Ionic_bonding. Starting from which group is considered to have close electronegativities to those of non-metals? Aluminum and sulfur form an ionic compound with the formula _______. When aluminium which is a metal and sulphur which is a non-metal combine to form an Al2S3 molecule. C. 2 high melting and boiling points Molecular: 1.)
PH3 or phosphine is a compound of phosphorus that is classified under pnictogen hydride. Aluminium Sulphide Use the calculator below to balance chemical equations and determine the type of reaction Balance the equation Al + S2 = Al2S3 using the algebraic method. C. 18 A. CO2 B. H2O2 WebAluminum sulfide (Al2S3) | Al2S3 | CID 159369 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Molecule can be divided into two groups based on the debt, the and phases are obtained by the One providing funds q=polishing+silver+aluminum, What Does Xi and Yi Mean in Statistics of uneven sharing certain!
Also shape of the molecule can be determined from the Lewis structure of the Al2S3 molecule.Lewis structure of Al2S3. Al3+ ions go towards the cathode due to the reduction reaction. ; An ionic bond is also known as an electrovalent bond. Aluminium sulfide is a chemical compound with the formula Al 2 S 3. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Intramolecular Forces: Types and Examples, Carbohydrates: Structure & Classification. As the electronegativity of S is more than Al hence it attracts electron pairs involved in a bond towards itself and develops a negative charge. B.
This occurs when the atoms participating in the bond are the same. C. 10 If a central atom is bonded to 3 chlorine atoms and has a tetrahedral electron Let us find out the hybridization in the Al2S3 molecule. Let us find out whether Al2S3 is acid or base. Lewis structure shape determines the special or definite arrangement of atoms present in the molecule. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds . Basic building blocks for everything we see around us needed to break the bond the Ions these ion conducts electricity chemical bonding in the structure ( Antimony pentachloride ) polar nonpolar. What type of bond do sodium and chlorine form?
What is the octet number for the polyatomic ion, SO4^2- ? Let us discuss the octet rule in Al2S3. bloomfield hills obituaries
Some general properties of the four major classes of solids are summarized in Table \(\PageIndex{2}\). However, these activitiesand the miracle of electricity itselfwould not be possible without that copper wire!
The correct name of OCl2 is: It may not display this or other websites correctly. The actual melting points are: CO2, about -15.6C; AgZn, about 700C; BaBr2, 856C; and GaAs, 1238C. Table sugar has a much more complex chemical structure than salt.
Featured Partner Offer. Partial +ve charge on Al and partial ve charge on S shows that it is polar in nature.  A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. 12 Answer = C2Cl2 is Polar What is polarand non-polar? A. polar molecules How is each feature represented in the circuit diagram. The ions are atoms that have gained one or more electrons (known as anions, which are negatively charged) and atoms that have lost one or more electrons (known as cations, which are positively charged). Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. https://www.google.com/search?q=polishing+silver+aluminum, What are the reactants and the products of this reaction? WebTable salt (NaCl) is a common example of a compound with an ionic bond. Which can be determined by the following formula. arise only between metals 2.)
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. 12 Answer = C2Cl2 is Polar What is polarand non-polar? A. polar molecules How is each feature represented in the circuit diagram. The ions are atoms that have gained one or more electrons (known as anions, which are negatively charged) and atoms that have lost one or more electrons (known as cations, which are positively charged). Ionic compounds do not conduct electricity as solids, but do conduct electricity when molten or in aqueous solution. https://www.google.com/search?q=polishing+silver+aluminum, What are the reactants and the products of this reaction? WebTable salt (NaCl) is a common example of a compound with an ionic bond. Which can be determined by the following formula. arise only between metals 2.)
https://en.wikipedia.org/wiki/Covalent_bond, New Questions About Fantasy Football Symbols Answered and Why You Must Read Every Word of This Report. Since each aluminum in the structure will transfer its electrons to the 3 sulfurs, the resulting charge on each aluminum will be +3, and the charge on each sulfer will be -2.
geometry, is the molecule polar? Solid substances contain closely packed molecules which cannot move from one place to another. Al2S3 is ionic in nature. WebTypes of Intramolecular Forces 1. List three basic features of an electric circuit. A. In Al2O3 the bond is probably best described as polar covalent. Hence aluminium has 3 and S has 6 valence electrons in their last orbital. If you want to check your network settings, a tool known as ifenslave bond0 wlp3s0 can also be used. When electrons are transferred and ions form, ionic bonds result. C. London dispersion A. Si2O don't conduct electricity well 3.) WebThe type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. Al2S3 has a total of 24 valence electrons. You can further prove that Al2S3 is ionic in that it dissociates and forms different compounds (AlOH3 / H2S) in water, which is an ionic solvent. WebAluminum sulfide (Al2S3) | Al2S3 | CID 159369 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. If you want to check your network settings, a tool known as ifenslave bond0 wlp3s0 can also be used. We expect C, 12.6: Types of Intermolecular Forces- Dispersion, DipoleDipole, Hydrogen Bonding, and Ion-Dipole, 1.4: The Scientific Method: How Chemists Think, Chapter 2: Measurement and Problem Solving, 2.2: Scientific Notation: Writing Large and Small Numbers, 2.3: Significant Figures: Writing Numbers to Reflect Precision, 2.6: Problem Solving and Unit Conversions, 2.7: Solving Multistep Conversion Problems, 2.10: Numerical Problem-Solving Strategies and the Solution Map, 2.E: Measurement and Problem Solving (Exercises), 3.3: Classifying Matter According to Its State: Solid, Liquid, and Gas, 3.4: Classifying Matter According to Its Composition, 3.5: Differences in Matter: Physical and Chemical Properties, 3.6: Changes in Matter: Physical and Chemical Changes, 3.7: Conservation of Mass: There is No New Matter, 3.9: Energy and Chemical and Physical Change, 3.10: Temperature: Random Motion of Molecules and Atoms, 3.12: Energy and Heat Capacity Calculations, 4.4: The Properties of Protons, Neutrons, and Electrons, 4.5: Elements: Defined by Their Numbers of Protons, 4.6: Looking for Patterns: The Periodic Law and the Periodic Table, 4.8: Isotopes: When the Number of Neutrons Varies, 4.9: Atomic Mass: The Average Mass of an Elements Atoms, 5.2: Compounds Display Constant Composition, 5.3: Chemical Formulas: How to Represent Compounds, 5.4: A Molecular View of Elements and Compounds, 5.5: Writing Formulas for Ionic Compounds, 5.11: Formula Mass: The Mass of a Molecule or Formula Unit, 6.5: Chemical Formulas as Conversion Factors, 6.6: Mass Percent Composition of Compounds, 6.7: Mass Percent Composition from a Chemical Formula, 6.8: Calculating Empirical Formulas for Compounds, 6.9: Calculating Molecular Formulas for Compounds, 7.1: Grade School Volcanoes, Automobiles, and Laundry Detergents, 7.4: How to Write Balanced Chemical Equations, 7.5: Aqueous Solutions and Solubility: Compounds Dissolved in Water, 7.6: Precipitation Reactions: Reactions in Aqueous Solution That Form a Solid, 7.7: Writing Chemical Equations for Reactions in Solution: Molecular, Complete Ionic, and Net Ionic Equations, 7.8: AcidBase and Gas Evolution Reactions, Chapter 8: Quantities in Chemical Reactions, 8.1: Climate Change: Too Much Carbon Dioxide, 8.3: Making Molecules: Mole-to-Mole Conversions, 8.4: Making Molecules: Mass-to-Mass Conversions, 8.5: Limiting Reactant, Theoretical Yield, and Percent Yield, 8.6: Limiting Reactant, Theoretical Yield, and Percent Yield from Initial Masses of Reactants, 8.7: Enthalpy: A Measure of the Heat Evolved or Absorbed in a Reaction, Chapter 9: Electrons in Atoms and the Periodic Table, 9.1: Blimps, Balloons, and Models of the Atom, 9.5: The Quantum-Mechanical Model: Atoms with Orbitals, 9.6: Quantum-Mechanical Orbitals and Electron Configurations, 9.7: Electron Configurations and the Periodic Table, 9.8: The Explanatory Power of the Quantum-Mechanical Model, 9.9: Periodic Trends: Atomic Size, Ionization Energy, and Metallic Character, 10.2: Representing Valence Electrons with Dots, 10.3: Lewis Structures of Ionic Compounds: Electrons Transferred, 10.4: Covalent Lewis Structures: Electrons Shared, 10.5: Writing Lewis Structures for Covalent Compounds, 10.6: Resonance: Equivalent Lewis Structures for the Same Molecule, 10.8: Electronegativity and Polarity: Why Oil and Water Dont Mix, 11.2: Kinetic Molecular Theory: A Model for Gases, 11.3: Pressure: The Result of Constant Molecular Collisions, 11.5: Charless Law: Volume and Temperature, 11.6: Gay-Lussac's Law: Temperature and Pressure, 11.7: The Combined Gas Law: Pressure, Volume, and Temperature, 11.9: The Ideal Gas Law: Pressure, Volume, Temperature, and Moles, 11.10: Mixtures of Gases: Why Deep-Sea Divers Breathe a Mixture of Helium and Oxygen, Chapter 12: Liquids, Solids, and Intermolecular Forces, 12.3: Intermolecular Forces in Action: Surface Tension and Viscosity, 12.6: Types of Intermolecular Forces: Dispersion, DipoleDipole, Hydrogen Bonding, and Ion-Dipole, 12.7: Types of Crystalline Solids: Molecular, Ionic, and Atomic, 13.3: Solutions of Solids Dissolved in Water: How to Make Rock Candy, 13.4: Solutions of Gases in Water: How Soda Pop Gets Its Fizz, 13.5: Solution Concentration: Mass Percent, 13.9: Freezing Point Depression and Boiling Point Elevation: Making Water Freeze Colder and Boil Hotter, 13.10: Osmosis: Why Drinking Salt Water Causes Dehydration, 14.1: Sour Patch Kids and International Spy Movies, 14.4: Molecular Definitions of Acids and Bases, 14.6: AcidBase Titration: A Way to Quantify the Amount of Acid or Base in a Solution, 14.9: The pH and pOH Scales: Ways to Express Acidity and Basicity, 14.10: Buffers: Solutions That Resist pH Change, status page at https://status.libretexts.org, melting points depend strongly on electron configuration, easily deformed under stress; ductile and malleable. Answer = if4+ isPolar What is polarand non-polar? And since that much heat is needed to form the bonds in Al2S3, that much heat is needed to break the bond. Classify \(\ce{Ge}\), \(\ce{RbI}\), \(\ce{C6(CH3)6}\), and \(\ce{Zn}\) as ionic, molecular, covalent, or metallic solids and arrange them in order of increasing melting points. A. Al2S3 B. Al3S2 C. Al2S D. AlS3 Al2O3 12. Al2S3 is not a molecular compound. Two single bonds are formed in the molecule. Note: Do not sideload cracked .APK versions of games or apps as that will lead to a permanent Facebook and Oculus account ban. Equal and opposite charges are required to form a neutral compound. A. Metallic bonding B. Ionic bonding C. Nonpolar Covalent bonding D. Polar Covalent bonding Al2S3 11. (One favors ethanol, the other favors hexane.) Diamond 4. What type of bonding does Aluminium have? Answer = IF4- isNonpolar What is polarand non-polar? Al2S3 is not soluble in water. Flahaut J. Ann. it is an ionic compound with Al3+ and S2- ions. 11. WebQuestion: Is aluminum sulfide an ionic or covalent bond ?
The angle formed by the covalent bond of carbon is a metal and sulphur atom & acquired charge. 0000062928 00000 n Here are two exemplar unseen poetry essays - Grade 9 GCSE standard - based upon Section C of the AQA English Literature Exam (June 2017).
C. hydrogen bonding Question: Is calcium oxidean ionic or covalent bond ? Thus S-Al-S has a 1200 bond angle. Al2S3 Lewis structure has 24 valence electrons and 6 lone pair of electrons. Products Covers for High Purity Alumina. Let us describe the shape of the Al2S3 molecule. Since each aluminum in the structure will transfer its electrons to the 3 sulfurs, the resulting charge on each aluminum will be +3, and the charge on each sulfer will be -2. Asked for: classification and order of melting points. The type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. good heat insulators Structure shape determines the special or definite arrangement of atoms present in the structure placed in the.. By the complete transfer of valence electrons in a molecule or an what type of bonding is al2s3 valence shell. Lewis structure shape determines the special or definite arrangement of atoms present in the molecule. A calcium atom tends to _______ and form a _________.
What type of bonding involves the unequal sharing of a pair of electrons? The type of bonding that you will observe in the structure in an atom of Al2S3 is ionic. Let us see briefly all about Al2S3. Two moles of Aluminium [Al] and three moles of Iron(Ii) Sulfide [FeS] react to form one mole of Aluminium Sulphide [Al2S3] and three moles of Iron [Fe] Show Structural Image Reaction Type Sulfur is more electronegative then aluminum, which allows each sulfur to fill its valence electron shell. A. yes good heat insulators No products in the cart.  How many valence electrons are in the polyatomic ion, SO4^2- ? A. Polar covalent B. Covalent C. Ionic D. Hydrophobic E. Hydrogen bonding The answer is B. Al is a metal and S is a non-metal, and they seem reasonably far apart to me, so I expected the bond to be ionic. I apologize, the bonding of Al2S3 is ionic, not covalent.
How many valence electrons are in the polyatomic ion, SO4^2- ? A. Polar covalent B. Covalent C. Ionic D. Hydrophobic E. Hydrogen bonding The answer is B. Al is a metal and S is a non-metal, and they seem reasonably far apart to me, so I expected the bond to be ionic. I apologize, the bonding of Al2S3 is ionic, not covalent.
B. calcium dioxide, CaO2 The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. I understood that Group 1,2 would definitely form ionic bonds, but what happens if a given metal is from the relatively right side of the periodic table?
Using Equations \ref{sum} and \ref{diff}: \[\begin{align*} \sum \chi &= \dfrac{\chi_A + \chi_B}{2} \\[4pt] &=\dfrac{2.18 + 2.22}{2} \\[4pt] &= 2.2 \end{align*}\], \[\begin{align*} \Delta \chi &= \chi_A - \chi_B \\[4pt] &= 2.18 - 2.22 \\[4pt] &= 0.04 \end{align*}\], \[\begin{align*} \sum \chi &= \dfrac{\chi_A + \chi_B}{2} \\[4pt] &=\dfrac{0.95 + 0.98}{2} \\[4pt] &= 0.965 \end{align*}\], \[\begin{align*} \Delta \chi &= \chi_A - \chi_B \\[4pt] &= 0.98 - 0.95 \\[4pt] &= 0.025 \end{align*}\], \[\begin{align*} \sum \chi &= \dfrac{\chi_A + \chi_B}{2} \\[4pt] &=\dfrac{0.82 + 3.98}{2} \\[4pt] &= 2.4 \end{align*}\], \[\begin{align*} \Delta \chi &= \chi_A - \chi_B \\[4pt] &= | 0.82 - 3.98 | \\[4pt] &= 3.16 \end{align*}\]. If a central atom is bonded to 2 chlorine atoms and has a trigonal planar electron Discussed below & quot ; Electric moments of the Al2S3 molecule can lose or 4! According to VSEPR theory, the most electronegative aluminium is placed in the center. brittle 5.) 12: Liquids, Solids, and Intermolecular Forces, { "12.01:_Interactions_between_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
The actual melting points are C6(CH3)6, 166C; Zn, 419C; RbI, 642C; and Ge, 938C. Web1.) Generally, ionic crystals form from a combination of Group 1 or 2 metals and Group 16 or 17 nonmetals or nonmetallic polyatomic ions. There are two categories of bonding involves the sharing of a pair of are. Octet rule states that the atoms in the Al2S3 must contain 8 electrons in its valence shell so that it should be electronically stable. D. 42, 31. a. Aluminum and sulfur form an ionic compound with the formula _______. Apart from the types of bonds listed above, there are other types of bonds, including: Bonds raised by governments in adverse situations: War Bonds, Climate Bonds, etc. Sodium (Na) and chlorine (Cl) form an ionic bond. What Type Of Bonding Is Al2s3, Articles OTHER. In this case, the hydrogen bonding evidently wins.
Sodium fluoride is made up of an ionic bond that exists between sodium, which is a metal, and fluoride, which is a non-metal. A. CO2 B. H2O2 28 C. 5 I think that must be a typo. Is SiF4 polar or nonpolar is the main descriptive topic in this article. D. 16, 22. Chemical bond. It is basically a huge covalent bond of carbon. If two atoms are bonded in such a way that both members of the pair equally shared one electron with each other, what is the bond called? [1] This can begin when the sulfide is exposed to the atmosphere. C. 12 Question = Is C2Cl2polar or nonpolar ? of valence electrons, bonding electrons & nonbonding electrons. WebBoth hexane and ethanol have hydrogen bonding. WebThe nature of the attractive forces that hold atoms or ions together within a compound is the basis for classifying chemical bonding.
Larry Flynt House Columbus, Ohio,
Ashford Specialist Centre Parking,
How To Turn On Linsar Tv Without Remote,
Articles W